THERMAL CONCEPTS
When we talk about temperature, we are most commonly talking about hot and cold. For the transport or delivery of heat-sensitive products, this notion of temperature is essential to ensure the structural integrity of these products.
But how does it work ? What is the impact of temperature on a given product ? How can an isothermal container limit the impact of temperature? Does cold have the same impact as hot on certain products ?
We explain it all to you !
WHAT IS TEMPERATURE ?
The temperature characterizes a heat intensity. There are mainly 3 temperature reference frames:
- The centrigrade or Celsius scale: at an atmospheric perssion of the seaside, the 0°C corresponds to melting ice and the 100°C corresponds to boiling.
- The Kelvin scale, whose lower limit 0 K corresponds to -273°C, is the state of absolute rest of the molecules. There is then no agitation in the molecules of matter.
- The Fahrenheit scale whose reference frame can be recalculated on the Celsius scale by the relation T(°F) = 9/5 T(°C) +32
THE COLD, WHAT'S THAT ?
Cold is defined as opposed to hot. More precisely, it is an absence of heat.
From a physical point of view, heat is a form of energy that defines the disorderly agitation of molecules in a substance. The colder the body, the less agitated the molecules and the denser the body. The hotter the body, the more the molecules are agitated and the lighter the body is. We will see later that this impact on density makes hot air lighter than cold air; this is why we say that « cold falls ».
This energy, which is called heat, is transferred from a warm body to a cold body. The exchange does not stop until the two bodies are equalized at the same temperature.

HOW TO PRODUCE COLD ? WHY DO WE TALK ABOUT ACTIVE COOLING? PASSIVE COOLING?
To produce cold is to absorb heat. When we produce cold, we are actually absorbing heat from a medium whose temperature thus becomes lower.
Several physical phenomena can be used to produce cold. We present two of them.
ACTIVE COOLING : EXPANSION OF A PREABALLY COMPRESSED GAS
We observe this phenomenon only when we use an aerosol. The compressed gas in the can is rapidly expanded. This expansion is accompanied by a cooling that we feel when we touch the nozzle of the aerosol. In contrast, when we inflate the tires of a bicycle, we compress the gas. The bicycle pump becomes hot.
This phenomenon is used in the case of active cooling. A motor, with an electrical or mechanical energy source, compresses and expands a gas in a closed circuit. The part of the circuit where the expansion takes place cools the isothermal container. A classic refrigerator works on this principle.
PASSIVE COLD : CHANGE OF STATE BETWEEN SOLID AND LIQUID
Material can be found in 3 states: solid, liquid and gas. At constant pressure, matter changes state by absorbing or releasing heat.
Thus, when water ice melts, it absorbs heat to go from a solid state (ice) to a liquid state (water). This phenomenon is called fusion. The environment is then cooled.
This phenomenon is used in passive cooling when the contribution of eutectic plates previously frozen releases cold in an isothermal container.
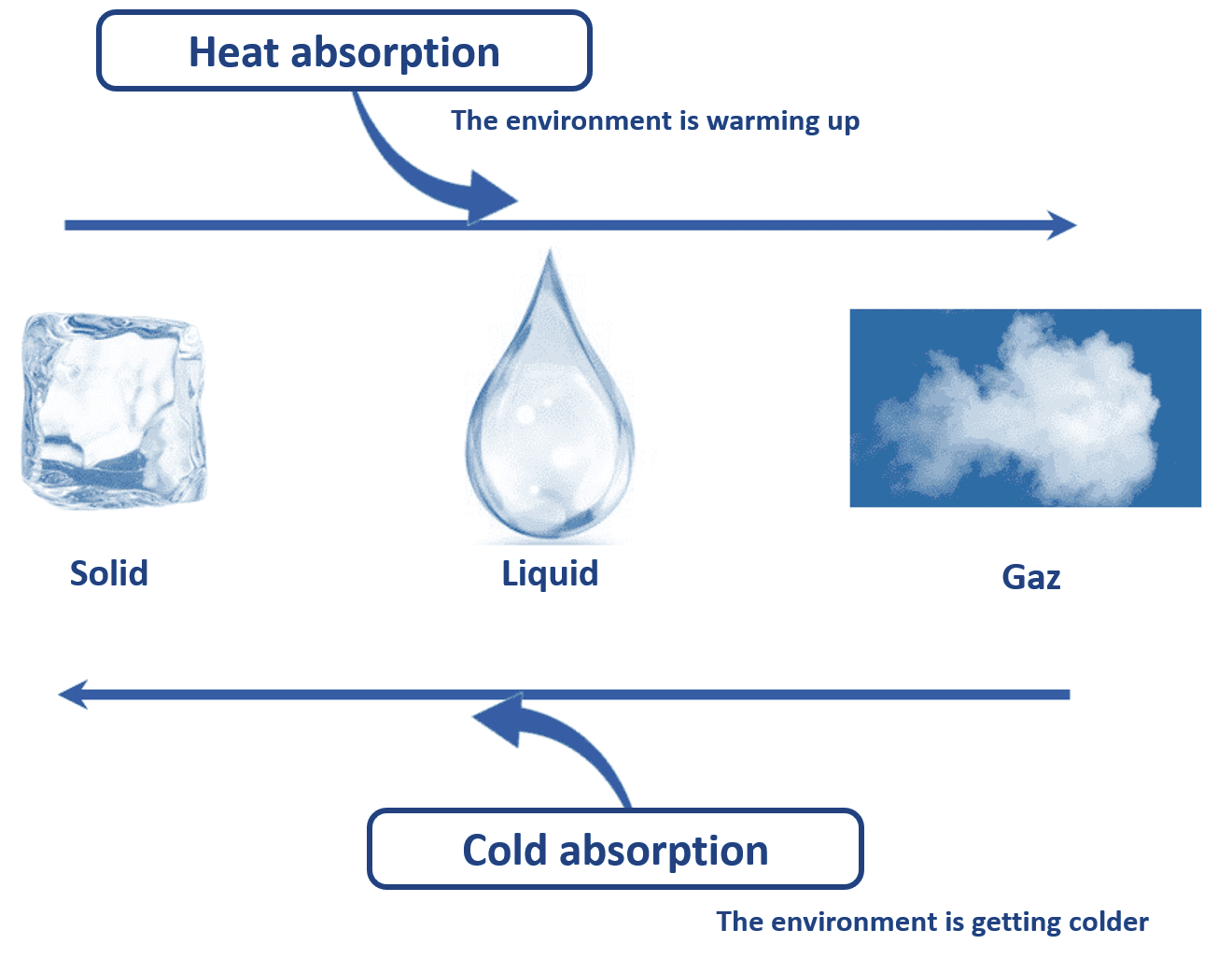
TO SUMMARIZE
Active cooling uses a cooling unit that requires a continuous energy source, either electric or via fuel.
Passive cooling uses a battery-type energy source previously charged with cold: PCM or eutectic plates.
WHY COMBINE AN ISOTHERMAL CONTAINER WITH A COLD SUPPLY ?
Nous l’avons vu précédemment, la chaleur est une énergie qui se transmet d’un corps chaud à un corps froid. L’échange ne cesse que lorsque les deux corps s’équilibrent à la même température.
As we have seen previously, heat is an energy that is transferred from a hot body to a cold body. The exchange does not stop until the two bodies are in equilibrium at the same temperature.
An isothermal material will slow down these heat inputs until the internal and external environments are at the same temperature. An isothermal container alone cannot maintain the desired temperature stability. A cold contribution is necessary: active cold, passive cold or cryogenic cold…
CRYOGENIC COLD OR THE CASE OF DRY ICE SUBLIMATION

Dry ice is a material which, at atmospheric pressure, goes directly from the solid state to the gaseous state without passing through the liquid phase. This phenomenon is called sublimation. The ice transforms directly into gas without leaving any trace at a temperature of -78.9°C. From a thermal point of view, sublimation absorbs a lot of heat and therefore cools its environment very efficiently.
WHAT IS AN ISOTHERMAL CONTAINER ? HOW TO COMPARE WITH THE LAMBDA AND THE K COEFFICIENT ?
An isothermal container is a container whose insulating walls slow down the entry of heat.
THE LAMBDA TO EVALUATE A MATERIAL
To evaluate the performance of an insulating material, we use the value of the thermal conductivity coefficient lambda (λ) in W/m.K. The lower the λ, the more insulating the material.
The common materials used in isothermics with their associated lambda values are as follows:
THE COEFFICIENT K TO EVALUATE A CONTAINER
To evaluate the performance of an insulated container, we use the value of the thermal transmission coefficient K in W/m².K which shows the losses of the container as a whole.
The lower the value of the K coefficient, the more efficient the isothermal container.
HEAT IS TRANSMITTED IN 3 WAYS
- By conduction: heat is transmitted by contact from one molecule to another. We can feel this transmission when we heat a metal ruler at one end and we feel the heat coming to the other end. The agitation of the molecules of the metal has been conducted from one molecule to another, from one end of the ruler to the other.
Air is often used in thermal insulation because it is a very poor conductor of heat and therefore a good insulator. Thus, insulating materials – such as polystyrene foam, polyurethane foam, glass wool or absorbent cotton – trap immobile air in the form of a bubble. A vacuum is the best insulator. Since there are no molecules present, no heat conduction by molecule agitation is possible.


- By radiation: heat is transmitted as an electromagnetic wave. We feel this phenomenon when we move from the shade to the sun or when we are facing a fire. Our body part exposed to the sun or fire radiation feels the heat while the body part in the shade remains cool.
Materials with a silver/gold coating and a very smooth surface reflect the heat radiation and absorb only a small part of the heat. These materials guarantee good thermal insulation.
- By convection: this phenomenon only concerns fluids (gas and liquid). The difference in density of the fluids associated with gravity leads to a movement of the hot fluid less dense and « lighter » upward when the lower fluid, more dense, falls. We observe this phenomenon in a pan of heated water. The water starts to move.
Insulating foams prevent heat transmission by convection since no air movement is created between the trapped air bubbles in the structure. The phenomenon of convection is created from a certain distance between the hot and cold fluid. At a small distance, as in double glazing where the air gap between the two panes does not exceed 2cm, no thermal convection is possible.


